Micro-Organ Device Mimics Organ Structures for Lab Testing
Health Medicine and Biotechnology
Micro-Organ Device Mimics Organ Structures for Lab Testing (MSC-TOPS-54)
Facilitates early stage in vivo-like drug screening without animal experimentation
Overview
NASA Johnson Space Center has developed the Micro-Organ Device (MOD) platform technology that serves as a drug screening system with human or animal cell micro-organs to supplement and reduce animal studies while potentially increasing the success of clinical trials. The technology was originally developed to evaluate pharmaceuticals in zero gravity to accelerate development and validation of countermeasures for humans in space as well as evaluate space and planetary stressors on a biological level.
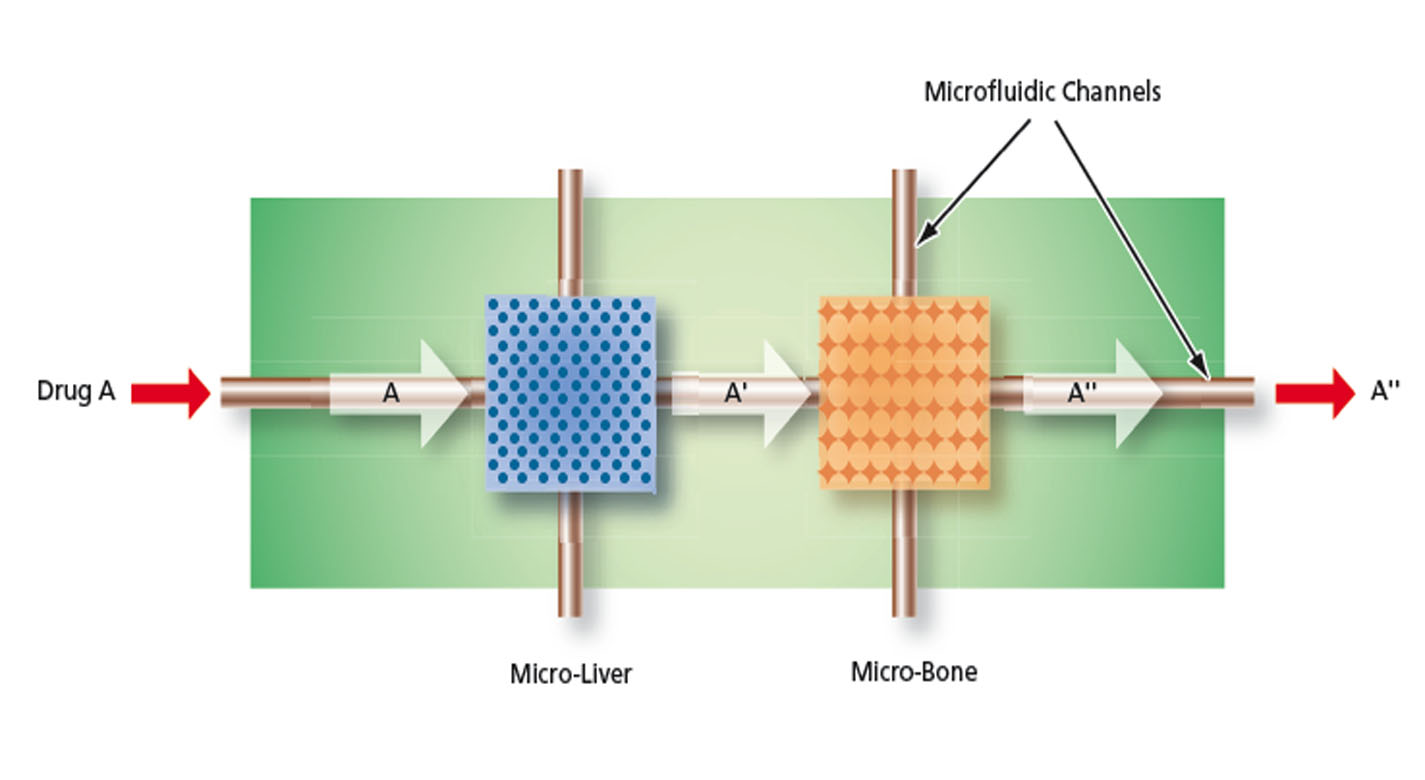
The MOD is a microfluidic device containing a variety of microstructures and assemblies of cells, all designed to mimic a complex in vivo microenvironment by modeling one or more in vivo micro-organ structures, the architectures and composition of the extracellular matrices in the organs of interest, and the in vivo fluid flows. The fully automated technology can be used to perform early stage in vivo drug screening without the use of animal experimentation, saving time, money, and resources.
This NASA Technology is available for your company to license and develop into a commercial product. NASA does not manufacture products for commercial sale.
The Technology
The MOD platform technology represents a small, lightweight, and reproducible in vitro drug screening model that could inexpensively mimic different mammalian tissues for a multitude of applications. The technology is automated and imposes minimal demands for resources (power, analytes, and fluids). The MOD technology uses titanium isopropoxide to bond a microscale support to a substrate and uses biopatterning and 3D tissue bioprinting on a microfluidic microchip to eliminate variations in local seeding density while minimizing selection pressure. With the MOD, pharmaceutical companies can test more candidates and concentrate on those with more promise therefore, reducing R&D overall cost.
This innovation overcomes major disadvantages of conventional in vitro and in vivo experimentation for purposes of investigating effects of medicines, toxins, and possibly other foreign substances. For example, the MOD platform technology could host life-like miniature assemblies of human cells and the effects observed in tests performed could potentially be extrapolated more readily to humans than could effects observed in conventional in vivo cell cultures, making it possible to reduce or eliminate experimentation on animals.
The automated NASA developed technology with minimal footprint and power requirements, micro-volumes of fluids and waste, high throughput and parallel analyses on the same chip, could advance the research and development for new drugs and materials.
Benefits
- 3D Tissue Models
- Quality Control
- Enhanced Accuracy
- Fully Automated
- Cost Efficient
- Minimal Resources Needed
Applications
- Pharmaceutical Drug Screening: Absorption, Distribution, Bioaccumulation, Metabolism, Efficacy, Toxicity
- Laboratory and Research Studies: Phamacokinetic and Pharmacodynamics studies
Technology Details
R. Chang, B. Starly, C. Culbertson, H. Holtorf, S. Gonda, W. Sun (2006) Development of an in vitro Micro-organ Model for Pharmacokinetic Microanalysis, Bioengineering Conference IEEE, 183-184 pages. doi: 10.1109/NEBC.2006.1629813
Similar Results

Miniature Bioreactor System for Cell Culture
The miniature bioreactor system was developed to provide the capabilities for NASA to perform cell studies in space and then provide results back to investigators on Earth with minimal tools and cost. The miniature bioreactor system has the potential to also be used on Earth as a laboratory bench-top cell culturing system without the need for expensive equipment and reagents.
The system can be operated under computer control to reduce the operator handling and to reduce result variations. The system includes a bioreactor, a fluid-handling subsystem, a chamber wherein the bioreactor is maintained in a controlled atmosphere and temperature, and control subsystems. The system can be used to culture both anchorage dependent and suspension cells (prokaryotic or eukaryotic cell types). Cells can be cultured for extended periods of time in this system, and samples of cells can be extracted and analyzed at specified intervals. The miniature bioreactor system for cell culturing has applications in pharmaceutical drug screening and cell culture studies.

Modular Container System Preserves Sample Integrity
The Astromaterials Acquisition and Curation Office (AACO) at NASA Johnson Space Center currently curates 500 milligrams of the regolith sample from the Asteroid Ryugu that was collected by the Japan Aerospace and Exploration Agency’s Hayabusa II spacecraft and returned to Earth in 2021. In September 2023, NASA’s OSIRIS-REx spacecraft returned 70 grams of regolith collected from the surface of Asteroid Bennu. These astromaterial sample collections are stored and handled in gloveboxes and desiccators that are continuously purged with ultrapure nitrogen in order to minimize contamination and alteration of extraterrestrial samples from terrestrial environments.
For collaborative astromaterial sample research conducted outside of the AACO, a need emerged for a sample container system suitable for global transport, capable of maintaining the same low-oxygen envi-ronment as laboratory gloveboxes. Thus, the MCS was developed. MSC’s of different sizes (2, 4, and 8-inch sample container models) have been developed to store contact pads and bulk samples from NASA missions, including the OSIRIS-REx Asteroid Bennu mission.
MCS’s are designed with seal profiles to prevent oxygen from seeping into the sample container. Additionally, the MCS uses a sample container form-factor that optimizes favorable nitrogen to oxygen gas ratios. The final prototypes were tested and verified using optochemical sensors to measure trace oxygen levels within the sealed containers.
The Modular Container System (MCS) could fill a critical gap in the existing high-purity logistics and storage market in its ability to provide a passively maintained, verifiable, multi-year, glovebox-level low-oxygen environment in a portable robust form-factor. Although this technology was originally developed for astromaterial transport and storage, commercial applications may also exist in biopharmaceutical/ bio-banking, microelectronics/ semiconductor, and other industries.

Portable Microscope
The handheld digital microscope features a 3D-printed chassis to house its hardware, firmware, and rechargeable Li-ion battery with built-in power management. It incorporates an internal stainless-steel cage system to enclose and provide mechanical rigidity for the optics and imaging sensor. To reduce the microscope’s size, yet retain high spatial resolution, engineers devised an optical light path that uniquely folds back on itself using high reflectivity mirrors, thus significantly reducing internal volume.
Imaging control and acquisition is performed using a secure web-based graphical user interface accessible via any wireless enabled device. The microscope serves as its own wireless access point thus obviating the need for a pre-existing network. This web interface enables multiple simultaneous connections and facilitates data sharing with clinicians, scientists, or other personnel as needed. Acquired images can be stored locally on the microscope server or on a removable SD card. Data can be securely downloaded to other devices using a range of industry standard protocols.
Although the handheld digital microscope was originally developed for in-flight medical diagnosis in microgravity applications, prototypes were thoroughly ground-tested in a variety of environments to verify the accurate resolve of microbial samples for identification and compo-sitional analysis for terrestrial field use. Owing to its portability, other applications demanding rapid results may include research, education, veterinarian, military, contagion disaster response, telemedicine, and point-of-care medicine.

Ionic Magnetic Resonance Tailors Animal Cells/Tissues
The apparatus comprises a randomized gravity vector multiphasic culture system with a self-feeding growth module, an optionally disposable nutrient module, and a removable AIMR chamber that delivers a pulsating multivariant field to the contents of the culture system. It produces overlapping or fluctuating alternating ionic magnetic resonance frequencies at one or more modal intervals ranging from about 7.8 Hz to about 59.9 Hz to the cell chamber. The apparatus may yield better regulation that can be manipulated to allow for increased rate of cell growth, faster differentiation, increased cell fidelity, and the induction or suppression of selective physiological genes involved in directing cellular differentiation and dedifferentiation.
The use of an AIMR field may provide a significant improvement over existing bioreactors, including pulsating electromagnetic field (PEMF) and time-variance electromagnetic field (TVEMF) cellular growth induced systems, in that AIMR incorporates the modulation of cellular transcription. The AIMR system utilizes pre-sterilized disposable modules and a removable alternating ionic magnetic resonance chamber, reducing the hazard for contamination, allowing scientists to implement physiological and homeostatic parameters similar to a naturally occurring physiological system.

Portable Medical Diagnosis Instrument
The technology utilizes four cutting-edge sensor technologies to enable minimally- or non-invasive analysis of various biological samples, including saliva, breath, and blood. The combination of technologies and sample pathways have unique advantages that collectively provides a powerful analytical capability. The four key technology components include the following: (1) the carbon nanotube (CNT) array designed for the detection of volatile molecules in exhaled breath; (2) a breath condenser surface to isolate nonvolatile breath compounds in exhaled breath; (3) the miniaturized differential mobility spectrometer (DMS) -like device for the detection of volatile and non-volatile molecules in condensed breath and saliva; and (4) the miniaturized circular disk (CD)-based centrifugal microfluidics device that can detect analytes in any liquid sample as well as perform blood cell counts. As an integrated system, the device has two ports for sample entry a mouthpiece for sampling of breath and a port for CD insertion. The breath analysis pathway consists of a CNT array followed by a condenser surface separating liquid and gas phase breath. The exhaled breath condensate is then analyzed via a DMS-like device and the separated gas breath can be analyzed by both CNT sensor array again and by DMS detectors.