Portable Medical Diagnosis Instrument
instrumentation
Portable Medical Diagnosis Instrument (TOP2-246)
Compact In-Flight Medical Diagnostic Technology for Deep-Space Missions
Overview
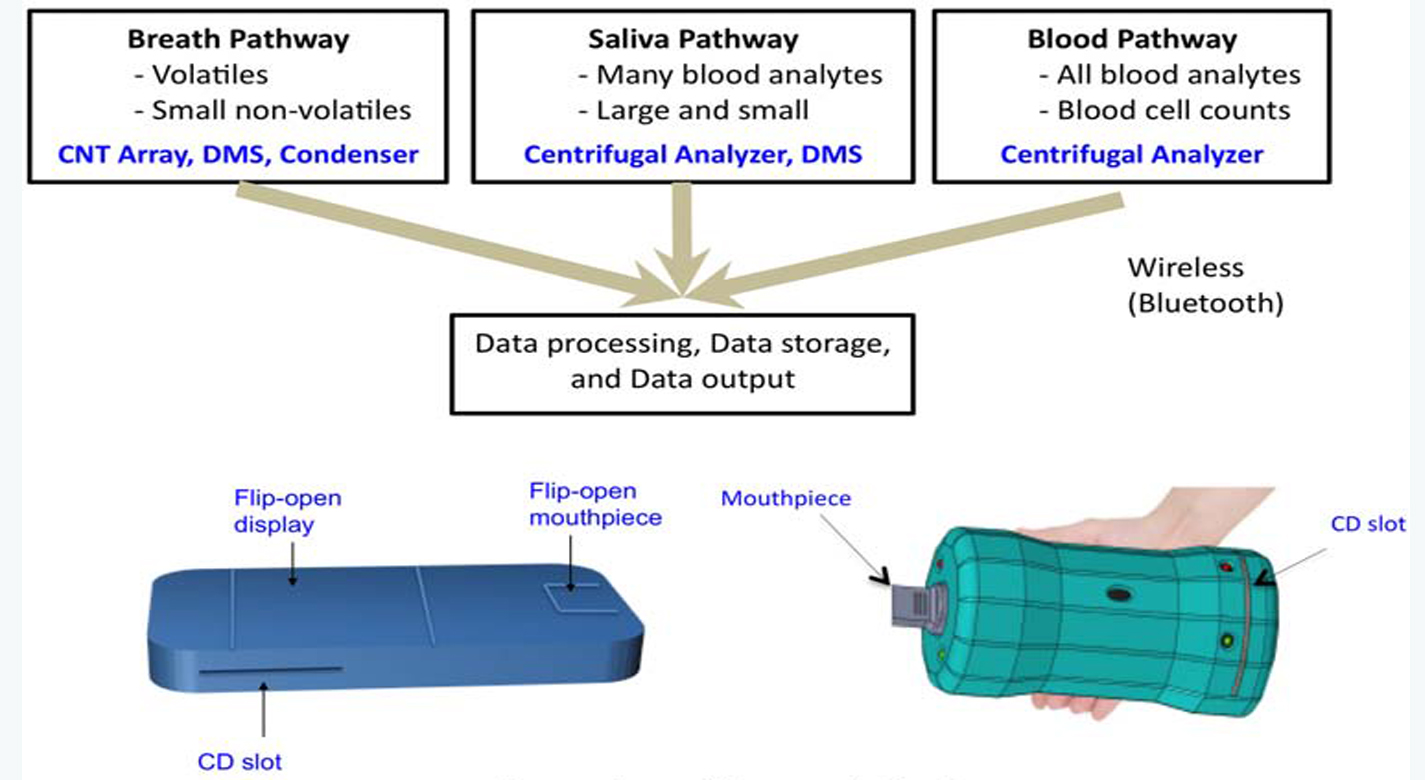
NASA has developed a novel technology strategy called "The NASA Analyzer" that would provide comprehensive in-flight medical diagnostic capability in a compact, hand-held device for human deep-space missions such as Mars. Key features of the technology include the ability to handle multiple sample types (breath, saliva, blood), and the ability to measure virtually any analyte, including future analytes as they emerge. The device provides both non-invasive and minimally invasive sampling capabilities, which will be required during long-duration exploration missions. Breath and saliva are fully non-invasive and can provide critical health assessment information very rapidly. From small blood samples, information about macromolecular analytes as well as blood cell counts can be obtained. The device consists of four cutting-edge technologies integrated into a single compact medical diagnostic tool with wireless (e.g., smart phone) capability. In addition to space applications, this innovative technology will very likely have important spin-offs in medicine and public health on Earth.
The Technology
The technology utilizes four cutting-edge sensor technologies to enable minimally- or non-invasive analysis of various biological samples, including saliva, breath, and blood. The combination of technologies and sample pathways have unique advantages that collectively provides a powerful analytical capability. The four key technology components include the following: (1) the carbon nanotube (CNT) array designed for the detection of volatile molecules in exhaled breath; (2) a breath condenser surface to isolate nonvolatile breath compounds in exhaled breath; (3) the miniaturized differential mobility spectrometer (DMS) -like device for the detection of volatile and non-volatile molecules in condensed breath and saliva; and (4) the miniaturized circular disk (CD)-based centrifugal microfluidics device that can detect analytes in any liquid sample as well as perform blood cell counts. As an integrated system, the device has two ports for sample entry a mouthpiece for sampling of breath and a port for CD insertion. The breath analysis pathway consists of a CNT array followed by a condenser surface separating liquid and gas phase breath. The exhaled breath condensate is then analyzed via a DMS-like device and the separated gas breath can be analyzed by both CNT sensor array again and by DMS detectors.
Benefits
- User-friendly, compact design, versatile, and hand-held device
- Graphical User Interface for data distillation, visualization, and overall system control to occur remotely
- Phone-sensor device possesses high sensitivity (parts-per-billion/parts per million), fast response time (seconds), high selectivity, both wireless and wired capabilities, and low power system
- DMS-type device provides analyte separation based on differences in ion mobility at high and low electric fields
- Centrifugal analyzer is a low-power, portable, has high analytical sensitivity and easy to use device for carrying out multiple immunoassays and cell-based analyses
- Non-invasive and minimal invasive specimens and does not require sample preparation prior to loading sample into the device
- Operates in real-time and generates multi-dimentional signals
- Software can be tailored to identify biomarkers of interest without the requirement of hardware modification
- Sensor can be "refreshed" for continuous use
Applications
- Space missions
- Health care industry
- Homeland security
- Field-testing and clinical diagnostics
- Military and disaster response
- Remote/harsh environments
Technology Details
instrumentation
TOP2-246
ARC-17171-1
Tore Straume, David J. Loftus, Jing Li, Matthew A. Coleman, Cristina E. Davis, Kathleen A. McMonigal, Matthew Piccini, and Anup K. Singh (2013) Biomarker-Detection Technologies for Comprehensive Medical Diagnosis During Deep-Space Missions, Recent Patents on Space Technology 3, 13-23.
Similar Results

Nanosensor Array for Medical Diagnoses
Many diseases are accompanied by characteristic odors. Their recognition can provide diagnostic clues, guide the laboratory evaluation, and affect the choice of immediate therapy. The study of the chemical composition of human breath using gas chromatography mass spectrometry (GC/MS) has shown a correlation between the volatile compounds and the occurrence of certain illnesses. The presence of those specific compounds can provide an indication of physiological malfunction and support the diagnosis of diseases. This condition requires an analytical tool with very high sensitivity for its measurement. A number of volatile compounds, so called biomarkers, are found in breath samples, normally at low parts per billion (ppb) levels. For example, the acetone in the exhaled breath from human with other biomarkers can indicate Type I diabetes. Usually, the concentration of the volatile compounds in human breath is very low and the background relative humidity is high, almost 100%. NASAs invention utilizes an array of chemical sensors combined with humidity, temperature, and pressure for real-time breath measurement to correlate the chemical information in the breath with the state and functioning of different human organs. This tool provides a non-invasive method for fast and accurate diagnosis at the medical point of care or at home. The sensor chip includes multisensors for a comprehensive measurement of chemical composition, temperature, humidity, and pressure/flow rate. The sensor data collected from this chip can be wired or wirelessly transmitted to a computer terminal at the doctors desk or hospital monitoring center. The sensor chip can be connected directly or via Universal serial bus (USB) to a cell phone for data transmission over a long distance and receive an instruction from a doctors office for an immediate therapy.

Portable Microscope
The handheld digital microscope features a 3D-printed chassis to house its hardware, firmware, and rechargeable Li-ion battery with built-in power management. It incorporates an internal stainless-steel cage system to enclose and provide mechanical rigidity for the optics and imaging sensor. To reduce the microscope’s size, yet retain high spatial resolution, engineers devised an optical light path that uniquely folds back on itself using high reflectivity mirrors, thus significantly reducing internal volume.
Imaging control and acquisition is performed using a secure web-based graphical user interface accessible via any wireless enabled device. The microscope serves as its own wireless access point thus obviating the need for a pre-existing network. This web interface enables multiple simultaneous connections and facilitates data sharing with clinicians, scientists, or other personnel as needed. Acquired images can be stored locally on the microscope server or on a removable SD card. Data can be securely downloaded to other devices using a range of industry standard protocols.
Although the handheld digital microscope was originally developed for in-flight medical diagnosis in microgravity applications, prototypes were thoroughly ground-tested in a variety of environments to verify the accurate resolve of microbial samples for identification and compo-sitional analysis for terrestrial field use. Owing to its portability, other applications demanding rapid results may include research, education, veterinarian, military, contagion disaster response, telemedicine, and point-of-care medicine.

Systems and methods employing nanomaterial sensors for detecting conditions impacting a Volatile Organic Compounds (VOCs) profile in breath
The technology involves a sophisticated system designed to detect conditions through the analysis of exhaled breath, utilizing an array of nanomaterial sensors fabricated upon a standard printed circuit board with interdigitated electrodes. These sensors are configured to interact with a sample gas that contains various Volatile Organic Compounds (VOCs) associated with a variety of biological conditions. Each sensor consists of nanomaterials, such as carbon nanotubes, composite nanotubes, nanoparticle-doped nanotubes, or polymer-coated nanotubes, all disposed on an electrically conductive structure. These sensors are highly sensitive to specific VOCs at a broad spectrum of concentrations, and each sensor generates a unique measurable electrical signal on interaction with VOCs in the breath that reflects the presence and concentration of specific components in the sample gas. The previously nanosensor diagnosis technology has been further developed to identify 64 specific formulations of nanomaterials that exhibit unique and varying sensitivities to VOCs, which enables unique response signatures to be developed for a wide range of VOCs. A single device may be developed using these principles to detect a variety of health conditions and diseases.

Micro-Organ Device Mimics Organ Structures for Lab Testing
The MOD platform technology represents a small, lightweight, and reproducible in vitro drug screening model that could inexpensively mimic different mammalian tissues for a multitude of applications. The technology is automated and imposes minimal demands for resources (power, analytes, and fluids). The MOD technology uses titanium isopropoxide to bond a microscale support to a substrate and uses biopatterning and 3D tissue bioprinting on a microfluidic microchip to eliminate variations in local seeding density while minimizing selection pressure. With the MOD, pharmaceutical companies can test more candidates and concentrate on those with more promise therefore, reducing R&D overall cost.
This innovation overcomes major disadvantages of conventional in vitro and in vivo experimentation for purposes of investigating effects of medicines, toxins, and possibly other foreign substances. For example, the MOD platform technology could host life-like miniature assemblies of human cells and the effects observed in tests performed could potentially be extrapolated more readily to humans than could effects observed in conventional in vivo cell cultures, making it possible to reduce or eliminate experimentation on animals.
The automated NASA developed technology with minimal footprint and power requirements, micro-volumes of fluids and waste, high throughput and parallel analyses on the same chip, could advance the research and development for new drugs and materials.

Portable Unit for Metabolic Analysis (PUMA)
PUMA represents a major breakthrough in portable metabolic analysis. It is a rugged, compact device that measures human metabolic function at rest, during exercise, in clinical settings, and in extreme environments. Metabolic measurements are a clinically proven method of monitoring cardiovascular health and fitness levels.
The PUMA headgear features NASA-developed sensors that evaluate six key metabolic functions. Specifically, PUMA measures oxygen and carbon dioxide partial pressure in addition to temperature, pressure, airflow, and heart rate. By placing sensors close to the mouth, PUMA can record up to 30 (or more) detailed measurements for each breath. From these measurements, PUMA computes metabolically relevant quantities of oxygen uptake, carbon dioxide output, minute ventilation, respiration rate, and heart rate. With additional software, the device computes heart rhythm, tidal volume, and alveolar and dead-space volumes. A small embedded computer controls and acquires data from all sensors at 10 hertz (Hz), performs calculations, and transmits data wirelessly to a remote computer. The PUMA sensors are low power, stable, and capable of operating in a range of environments, including very high and low pressures as well as high- and low-oxygen environments. This portable device provides real-time measurements that are just as accurate as the large stationary metabolic carts used in hospitals. PUMA can be used not only in clinical settings but also in the extreme/remote environments of space, aviation, underwater, and deep underground. Because it detects real-time dangerous drops in oxygen, it can ensure astronaut cardiovascular health; predict the onset of hypoxia in pilots, divers, and first responders; and advance chronic pulmonary disease monitoring and athletic training.



