Bio-Magnetic Device To Enhance Mammalian Tissue Repair
health medicine and biotechnology
Bio-Magnetic Device To Enhance Mammalian Tissue Repair (MSC-TOPS-112)
Portable sleeve uses electromagnetism to manipulate blood vessels
Overview
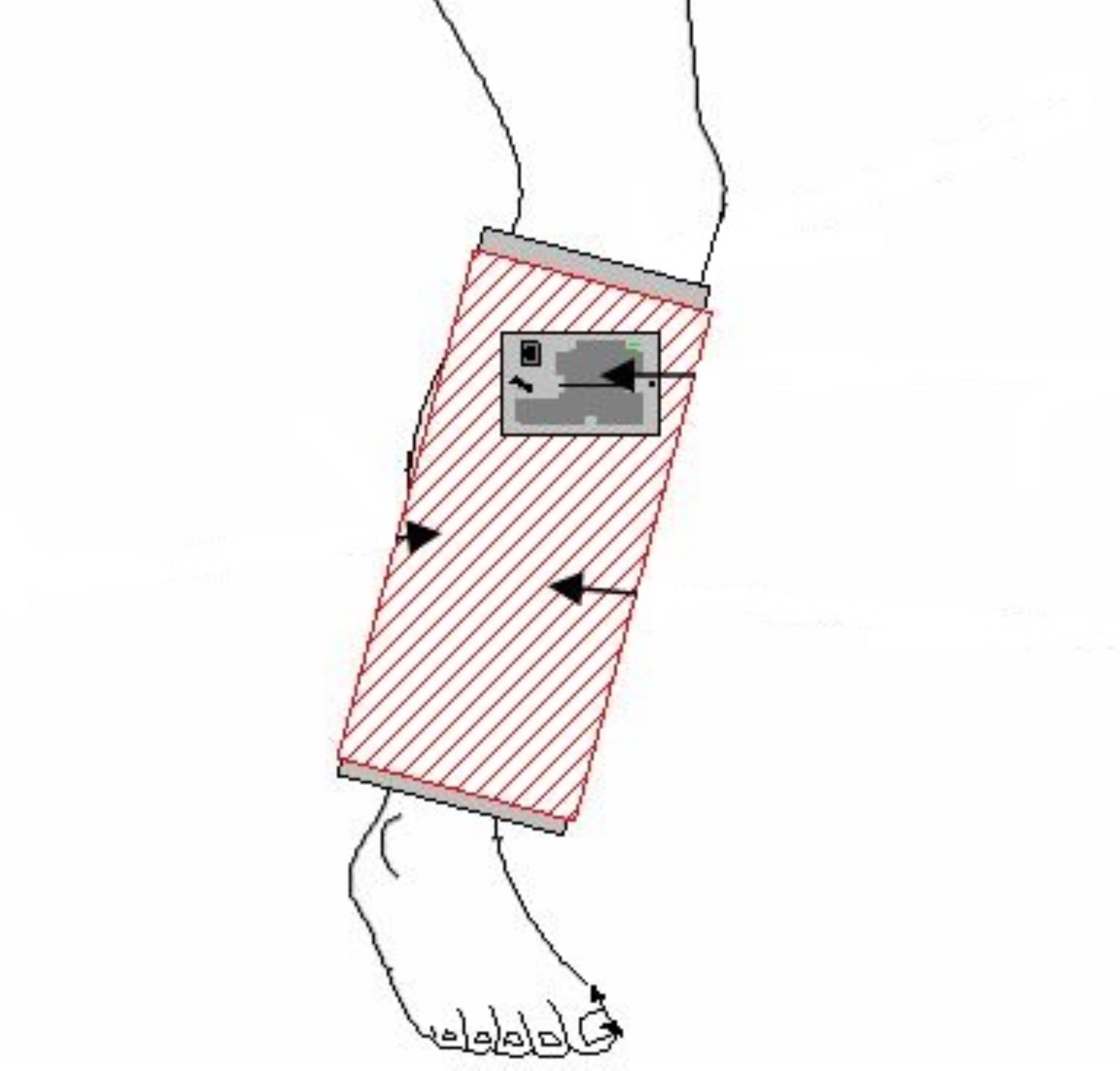
Innovators at NASA Johnson Space Center have designed a therapeutic device that applies a time-varying electromagnetic force to damaged mammalian tissue and is intended to enhance healing. The device is mainly comprised of a sleeve that encircles the affected appendage and operates using an internal electromagnetic coil. The sleeve encircles the target appendage and applies a carefully titrated electromagnetic field for a predetermined amount of time using only a compact commercially available electrical generator, and a 9-volt battery.
The device is easily portable and intends to mend soft tissue and enhance the repair of bone fractures.
The Apparatus for Enhancing Tissue Repair in Mammals is technology readiness level (TRL) 2 (technology concept and/or application formulated) and is now available for patent licensing. Please note that NASA does not manufacture products itself for commercial sale.
The Technology
Most magnetic therapy research and resulting devices have centered around pulsed unidirectional bioelectric systems. The technology available here for licensing utilizes a square-wave time-varying electrical current, which generates an electromagnetic field, via a wound coil incorporated into a sleeve and encircles the affected appendage. An external and commercially available time-varying compact electrical generator connects to the wound coil within the sleeve and is powered by a 9-volt battery.
Prior industry attempts to use electromagnetic therapy on mammalian tissue have historically applied higher than necessary levels of electromagnetism, typically at 50 gauss or more. Researchers found that by inducing a Fourier-curve, time-varying electromagnetic wave at levels within 0.05 0.5 gauss for a pre-determined time-period, was optimum to achieve successful mammalian tissue regeneration.
It is theorized that magnetic fields can alter the flow of positively charged calcium ions that interact with the muscles around small blood vessels causing them to relax. This effect in turn, causes constricted blood vessels to dilate, and dilated blood vessels to constrict. Depending upon the type of injury, enhanced tissue repair may occur through the suppression of inflammation, or the increase in blood flow.

Benefits
- Portable: small form-factor requires minimal space for implementation
- Lightweight: requires minimal effort to transport
- Inexpensive: main component generator is commercially available
- Low energy consumption: system runs off of simple 9-volt battery
- Versatile: main component sleeve can be adapted to various mammalian appendages
- Easy to assemble/use: system requires only two connections and two switches
Applications
- Contemporary Medicine: may expedite healing of hard and soft tissue in humans
- Veterinary Medicine: may expedite healing of hard and soft tissue in other mammals
|
Tags:
|
Similar Results

Electroactive Material for Wound Healing
An electroactive device is applied to an external wound site. This method utilizes generated low level electrical stimulation to promote the wound healing process while simultaneously protecting it from infection. The material is fabricated from polyvinylidene fluoride, or PVDF, a thermoplastic fluoropolymer that is highly piezoelectric when poled. The fabrication method of the electroactive material is based on a previous Langley invention of an apparatus that is used to electrospin highly aligned polymer fiber material. A description of the fabrication method can be found in the technology opportunity announcement titled "NASA Langley's Highly Electrospun Fibers and Mats," which is available on NASA Langley's Technology Gateway.

Noninvasive Therapy for Cartilage Regeneration
Research has shown that exposure of mammalian cartilage and bone tissue to tuned magnetic fields modifies genetic regulation at a cellular level. PEMF therapy relies on modulation and resonance of weak metals (ions) such as Ca2+, K+, Li+, and Mg2+ which can be made to move at the sub-cellular level when exposed to magnetic flux. This NASA technology is a device and method for modifying genetic regulation of cartilage and bone in response to PEMF therapy and may serve as the basis for development of novel therapies for cartilage diseases.
In initial studies, cultured human chondrocyte cells (HCH) from patients with early-stage osteoarthritis were exposed to PEMF stimulation using a variety of tuned electro-magnetic pulse characteristics such as flux magnitude, slew rates, rise and fall times, frequency, wavelength, and duty cycle. Waveforms used in testing were monophasic, bi-phasic, square, sinusoidal, and triangular in nature. Frequencies were generally low, ranging from 6-500 Hz, and the waveforms used high rising and falling slew rates on the order of Tesla/sec, promoting pulses or bursts.
Cellular catabolic and anabolic gene expression analyses comprised of fold-change (in expression) were accomplished by a survey of 47,000 human genes using an AFFYMETRIX Gene Array. Results show that variation of waveform used in PEMF therapies, independent of flux intensity, influences genetic regulation of HCH from patients with early-stage osteoarthritis.

Electroactive Scaffold
Current scaffold designs and materials do not provide all of the appropriate cues necessary to mimic in-vivo conditions for tissue engineering and stem cell engineering applications. It has been hypothesized that many biomaterials, such as bone, muscle, brain and heart tissue exhibit piezoelectric and ferroelectric properties. Typical cell seeding environments incorporate biochemical cues and more recently mechanical stimuli, however, electrical cues have just recently been incorporated in standard in-vitro examinations. In order to develop their potential further, novel scaffolds are required to provide adequate cues in the in-vitro environment to direct stem cells to differentiate down controlled pathways or develop novel tissue constructs. This invention is for a scaffold that provides for such cues by mimicking the native biological environment, including biochemical, topographical, mechanical and electrical cues.

Upper Body Robotic Exoskeleton
NASA's soft, portable, wearable robotic device is "plug and play" - it includes all necessary electronics, actuation, software, and sensors required to achieve augmented limb movement. The garment is designed such that the human-robot interface distributes load across the torso, maximizing user comfort. Donning and doffing is simple, as the device lowers over the head, straps to the torso via Velcro, and possesses adjustable custom arm cuffs. Actuators are housed in the back of the garment, which pull custom conduit-tendon-based systems attached to the limb at optimized locations, causing the joint of interest to move to the specified orientation. Force sensing is employed to enable optimal control of the limb, measuring user-applied force to maintain commanded joint orientations. Integrated electronics and software provide power distribution, safety monitoring, data transfer and data logging.
NASA's garment has multiple modes of operation. In active assist mode, shoulder abduction and flexion, and elbow flexion, may be commanded either simultaneously via coordinated control or individually while holding position/orientation of the other joints. In passive assist mode, the user can freely move the limb while the system provides minimal torque to the shoulder and elbow.
The upper body robotic exoskeleton is at a TRL 6 (system/subsystem prototype demonstration in a relevant environment) and it is now available for your company to license and develop into a commercial product. Please note that NASA does not manufacture products itself for commercial sale.

Ionic Magnetic Resonance Tailors Animal Cells/Tissues
The apparatus comprises a randomized gravity vector multiphasic culture system with a self-feeding growth module, an optionally disposable nutrient module, and a removable AIMR chamber that delivers a pulsating multivariant field to the contents of the culture system. It produces overlapping or fluctuating alternating ionic magnetic resonance frequencies at one or more modal intervals ranging from about 7.8 Hz to about 59.9 Hz to the cell chamber. The apparatus may yield better regulation that can be manipulated to allow for increased rate of cell growth, faster differentiation, increased cell fidelity, and the induction or suppression of selective physiological genes involved in directing cellular differentiation and dedifferentiation.
The use of an AIMR field may provide a significant improvement over existing bioreactors, including pulsating electromagnetic field (PEMF) and time-variance electromagnetic field (TVEMF) cellular growth induced systems, in that AIMR incorporates the modulation of cellular transcription. The AIMR system utilizes pre-sterilized disposable modules and a removable alternating ionic magnetic resonance chamber, reducing the hazard for contamination, allowing scientists to implement physiological and homeostatic parameters similar to a naturally occurring physiological system.