Solid State Carbon Dioxide (CO2) Sensor
sensors
Solid State Carbon Dioxide (CO2) Sensor (TOP2-292)
Miniaturized, chip size solid state CO2 sensor
Overview
Detection of Carbon Dioxide (CO2) is very important for environmental, health, safety and space applications. CO2 is a harmful pollutant at higher concentrations due to its ability to displace oxygen in large concentrations. Current commercial sensors for CO2 have issues and shortcomings particular with precision at different temperatures, pressures and high humidity levels. NASA Ames has developed a novel solid state, CO2 sensor configured for sensitive detection of CO2 having a concentration within the range of about 100 Parts Per Million (ppm) and 10,000 ppm in both dry conditions and high humidity conditions. The composite sensing material comprises Oxidized Multi-Walled Carbon Nanotubes (O-MWCNT) and a metal oxide. The composite sensing material has an inherent resistance and corresponding conductivity that is chemically modulated as the level of CO2 increases. The CO2 sensor can be easily integrated into existing electronic circuitry and hardware configurations, including the hardware of a mobile computing device, such as a smart phone or tablet device.
The Technology
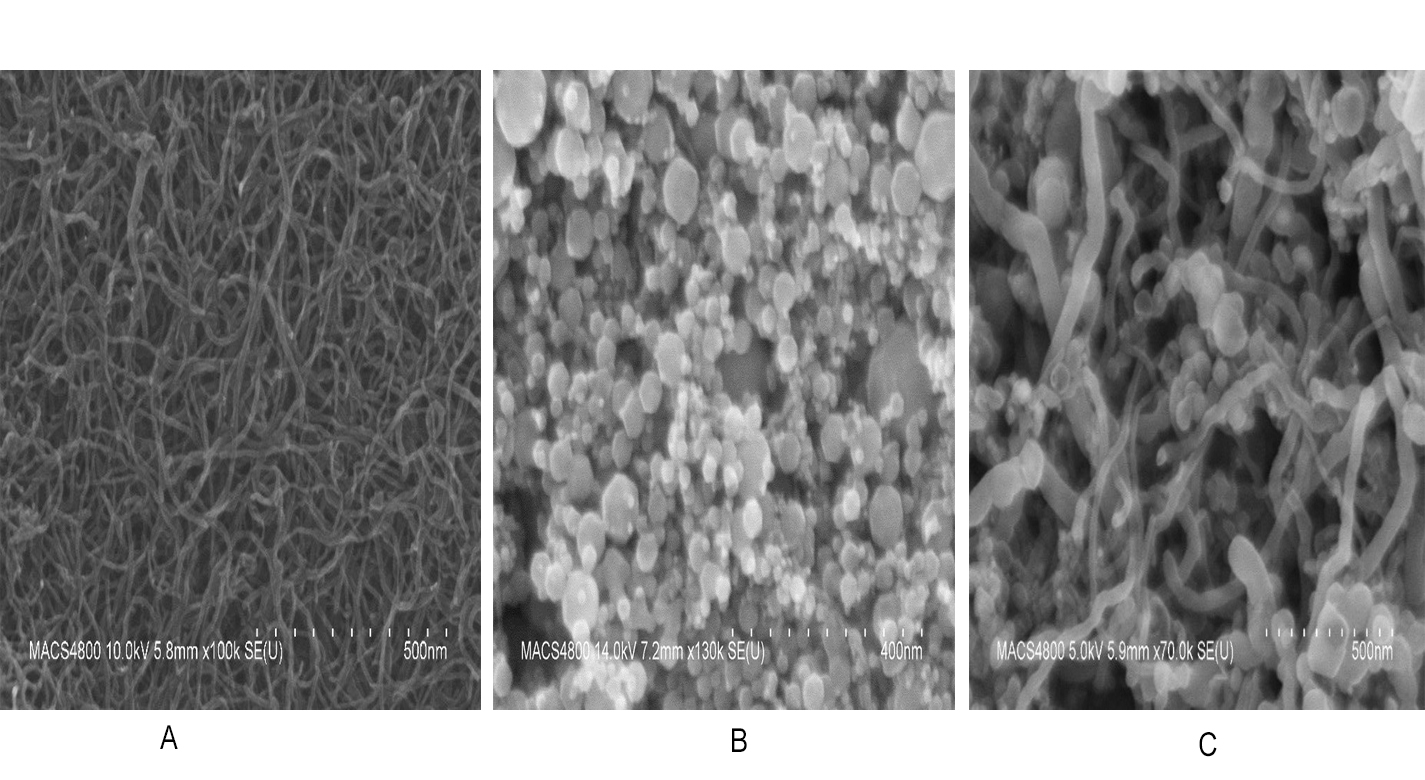
The technology is a solid state, Carbon Dioxide (CO2) sensor configured for sensitive detection of CO2 having a concentration within the range of about 100 Parts per Million (ppm) and 10,000 ppm in both dry conditions and high humidity conditions (e.g., > 80% relative humidity). The solid state CO2 sensor achieves detection of high concentrations of CO2 without saturation and in both dynamic flow mode and static diffusion mode conditions. The composite sensing material comprises Oxidized Multi-Walled Carbon Nanotubes (O-MWCNT) and a metal oxide, for example O-MWCNT and iron oxide (Fe2O3) nanoparticles. The composite sensing material has an inherent resistance and corresponding conductivity that is chemically modulated as the level of CO2 increases. The CO2 gas molecules absorbed into the carbon nanotube composites cause charge-transfer and changes in the conductive pathway such that the conductivity of the composite sensing material is changed. This change in conductivity provides a sensor response for the CO2 detection. The solid state CO2 sensor is well suited for automated manufacturing using robotics and software controlled operations. The solid state CO2 sensor does not utilize consumable components or materials and does not require calibration as often as conventional CO2 sensors. Since the technology can be easily integrated into existing programmable electronic systems or hardware systems, the calibration of the CO2 sensor can be automated.

Benefits
- Accurate and rapid response - quickly provides accurate readings (in seconds) at different temperatures and pressures
- High CO2 sensitivity: CO2 can be measured in the 100-10,000 ppm range
- Operates at room temperature: Unlike standard metal oxide sensors which must operate at ~300 Celsius or higher, these sensors operate at room temperature (~25 Celsius)
- Small footprint: Chips can be made 0.5 cm x 0.5 cm x 3 mm size with multiple sensors per chip
- Light weight: Sensor only weighs a few grams
- Low cost and low power: Less than 50 microwatts are required for power, and the sensor functions on a change in resistivity
- Easy to integrate: Can be easily coupled with existing programmable electronic or hardware systems
- Sensor is solid state; so extra materials are required to maintain operation
- Provides in-situ monitoring; and calibration of the CO2 sensor that can be automated
Applications
- Wearable sensor for environmental monitoring
- Astronaut atmospheric monitoring; CO2 in a space suit, particularly within the astronauts' helmets
- Modified atmospheres or closed crew cabin to continuously monitor real-time CO2 concentrations for permissible exposure limit levels; space-medicine
- Determining the catalyzing efficiency of the CO2 splitting process; in-situ resource utilization of CO2 on Mars
- Aerospace industry - for cabin air monitoring on the airplane
- Medical diagnosis and monitoring
- Indoor air quality
- Stowaway detection
- Monitoring landfill gas
- Confined spaces; Cryogenics; Ventilation management, Mining industry
- Rebreathers (SCUBA)
- Cellar and gas stores; Marine vessels, and Greenhouses
- Food industry
- Monitoring global warming
- CO2 scrubber industry
Technology Details
sensors
TOP2-292
ARC-18097-1
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6767332/
|
Tags:
|
Similar Results

Room temperature oxygen sensors
NASA Ames has developed very small-sized oxygen sensors made of a graphene and titanium dioxide (TiO2) hybrid material. With ultraviolet (UV) illumination, these sensors are capable of detecting oxygen (O2) gas at room temperature and at ambient pressure. The sensors are able to detect oxygen at concentrations ranging from about 0.2% to about 10% by volume under 365nm UV light, and at concentrations ranging from 0.4% to 20% by volume under short wave 254nm UV light. These sensors have fast response and recovery times and can also be used to detect ozone.
This unique room temperature O2 sensor provides significant advantages in O2 sensing applications, especially those applications where high operating temperature requirements cannot be met, or would result in inefficient manufacturing processes. Since graphene is not intrinsically responsive to O2, and TiO2 is not responsive to oxygen at room temperature, the materials are first synthesized as a hybrid material. The synthesized graphene- TiO2 hybrid material is then ultrasonicated and then drop-casted onto a series of Interdigitated Electrodes (IDE) to form the sensors.
Ultrasonication ensures effective charge transfer at the graphene- TiO2 interphase. The graphene and the titanium dioxide may be present in the composite material in different ratios to ensure optimal oxygen detection. It is the combination of graphene with TiO2 that yields a semiconducting material capable of O2 sensing at room-temperature operation.

Gas Sensors Based on Coated and Doped Carbon Nanotubes
A typical sensor device includes a set of interdigitated microelectrodes fabricated by photolithography on silicon wafer or an electrically insulating substrate. In preparation for fabricating the SWCNT portion of such a sensor, a batch of treated (coated or doped) SWCNTs is dispersed in a solvent. The resulting suspension of SWCNTs is drop-deposited or injected onto the area containing the interdigitated electrodes. As the solvent evaporates, the SWCNTs form a mesh that connects the electrodes. The density of the SWCNTs in the mesh can be changed by varying the concentration of SWCNTs in the suspension and/or the amount of suspension dropped on the electrode area. To enable acquisition of measurements for comparison and to gain orthogonality in the sensor array, undoped SWCNTs can be similarly formed on another, identical set of interdigitated electrodes. Coating materials tested so far include chlorosulfonated polyethylene. Dopants that have been tested include Pd, Pt, Au, Cu and Rh nanoparticle clusters. To date, the sensor has been tested for NO2, NH3, CH4, Cl2, HCl, toluene, benzene, acetone, formaldehyde and nitrotoulene.

Solar Powered Carbon Dioxide (CO2) Conversion
This technology consists of a photoelectrochemical cell composed of thin metal oxide films. It uses sunlight (primarily the ultraviolet (UV), visible and Infrared (IR) portions)) and inexpensive titanium dioxide composites to perform the reaction. The device can be used to capture carbon dioxide produced in industrial processes before it is emitted to the atmosphere and convert it to a useful fuel such as methane. These devices can be deployed to the commercial market with low manufacturing and materials costs. They can be made extremely compact and efficient and used in sensor and detector applications.

Miniaturized Laser Heterodyne Radiometer
This instrument uses a variation of laser heterodyne radiometer (LHR) to measure the concentration of trace gases in the atmosphere by measuring their absorption of sunlight in the infrared. Each absorption signal is mixed with laser light (the local oscillator) at a near-by frequency in a fast photoreceiver. The resulting beat signal is sensitive to changes in absorption, and located at an easier-to-process RF frequency. By separating the signal into a RF filter bank, trace gas concentrations can be found as a function of altitude.

Hybrid carbon nanotube-gold nanoparticle composite for Nitric Oxide (NO) detection
A hybrid thin film is fabricated by a simple drop-casting method. Functionalized single-walled carbon nanotubes (SWCNTs) and gold nanoparticles (AuNPs) with a diameter of ≈15 nm are drop-casted onto a printed circuit board (PCB) substrate equipped with interdigitated electrodes. The addition of AuNPs to the carbon nanotube networked films enhance sensitivity and lower the detection limit to low parts-per-billion (ppb) concentrations. The gold particle to carbon nanotube ratio is optimized to find the optimum gold nanoparticle loading.
The composite films were tested in both air and nitrogen environments across a wide relative humidity range (0-97%), which is suitable for dissolved Nitric Oxide (NO) detection in sea water for oceanographic study and for human breath analysis in medical diagnosis. The sensors exhibited high selectivity, particularly to NO, outperforming other tested gases. Notably, the sensor reliably detected NO at 10 ppb levels with response times within 10 seconds and recovery time around 1 minute, showcasing excellent reproducibility across sensors and operational efficiency within diverse humidity conditions.



