Search
Health Medicine and Biotechnology

Human-Powered Ventilator
In space there are a limited number of care providers, and those providers are not always clinicians with extensive medical training. Space travel also has limited room to provide care and limited consumables. The Human-Powered Ventilator is compact, portable, and easy to assemble. It is designed so that users can implement hand and arm movements to pump the bellows between two hinged, clamshell-like panels back and forth to provide positive pressure ventilation to the patient. A light spring is incorporated into the design to assist in expanding the bellows, drawing air out of the patient’s lungs, and reducing the physical load on the operator without compromising the tactile feel necessary for proper usage. The airflow can be supplemented with prescribed medical vapors, oxygen, etc. via standard industry fittings.
The Human-Powered Ventilator is TRL 6 (system/subsystem prototype has been demonstrated in a relevant environment) and it is now available for your company to license. Please note that NASA does not manufacture products itself for commercial sale.
Environment

Modular Container System Preserves Sample Integrity
The Astromaterials Acquisition and Curation Office (AACO) at NASA Johnson Space Center currently curates 500 milligrams of the regolith sample from the Asteroid Ryugu that was collected by the Japan Aerospace and Exploration Agency’s Hayabusa II spacecraft and returned to Earth in 2021. In September 2023, NASA’s OSIRIS-REx spacecraft returned 70 grams of regolith collected from the surface of Asteroid Bennu. These astromaterial sample collections are stored and handled in gloveboxes and desiccators that are continuously purged with ultrapure nitrogen in order to minimize contamination and alteration of extraterrestrial samples from terrestrial environments.
For collaborative astromaterial sample research conducted outside of the AACO, a need emerged for a sample container system suitable for global transport, capable of maintaining the same low-oxygen envi-ronment as laboratory gloveboxes. Thus, the MCS was developed. MSC’s of different sizes (2, 4, and 8-inch sample container models) have been developed to store contact pads and bulk samples from NASA missions, including the OSIRIS-REx Asteroid Bennu mission.
MCS’s are designed with seal profiles to prevent oxygen from seeping into the sample container. Additionally, the MCS uses a sample container form-factor that optimizes favorable nitrogen to oxygen gas ratios. The final prototypes were tested and verified using optochemical sensors to measure trace oxygen levels within the sealed containers.
The Modular Container System (MCS) could fill a critical gap in the existing high-purity logistics and storage market in its ability to provide a passively maintained, verifiable, multi-year, glovebox-level low-oxygen environment in a portable robust form-factor. Although this technology was originally developed for astromaterial transport and storage, commercial applications may also exist in biopharmaceutical/ bio-banking, microelectronics/ semiconductor, and other industries.
Optics

Portable Integrated Fourier Ptychographic Microscope
NASA’s integrated, portable FPM device combines advanced optical microscopy with AI in a compact form factor. At its core, the system uses a Raspberry Pi camera module equipped with an 8-megapixel sensor and a 3-mm focal-length lens, achieving approximately 1.5× magnification. Illumination is provided by a Unicorn HAT HD LED array positioned 65mm below the sample stage, creating a synthetic numerical aperture of 0.55. All these components are controlled by an NVIDIA Jetson Nano board, which serves as the system's embedded AI computing platform. NASA’s portable FPM device can be integrated with a microfluidic system for sub-micron imaging of liquid samples.
In addition to its portability, what sets NASA’s integrated FPM device apart is its integration of deep learning capabilities. The invention has two modes – normal mode and deep learning mode. In its normal mode, the system captures data and performs intensity and 3D phase analysis using traditional FPM methods. The deep learning mode enhances this base functionality by employing neural networks for image reconstruction and system optimization. The AI system automatically detects when samples are out of focus and can either mechanically or digitally adjust to the correct focal plane. To achieve near real-time monitoring, deep learning models significantly reduce data acquisition time by selectively using only a portion of the LED array and provide fast reconstruction capabilities leveraging training on specific sample types.
While originally designed for imaging biosignature motility in liquid samples for spaceflight applications, this NASA innovation can image many different types of samples, and is not limited to biological specimens. The ability to operate this system in the field further broadens use-cases.
Power Generation and Storage

Next Generation Li-Ion Calorimeter
Among the enhancements reflected in the Next Generation Li-ion Calorimeter is a rigidly wired system that allows direct mounting of thermocouples into key component locations to better capture thermal signature data during testing and improve thermocouple reliability. The ejecta mating chambers have also been modified for better thermal containment and easier system disassembly. Additionally, the system facilitates an easier access, user-friendly Destructive Physical Analysis (DPA) process between uses, and reflects durability improvements in the face of repetitive heat cycling.
A clean-sheet redesign was undertaken to create a configurable insula-tion case with an interchangeable “window” section, tailored to the ex-perimental environment. For NASA’s Energy Systems Test Area (ESTA) evaluation, a window with the original foam is installed to maintain ther-mal insulation performance. In contrast, for synchrotron experiments, this section is replaced with an aluminum window that eliminates foam-related X-ray scattering. This modification has substantially improved X-ray radiography resolution, enabling clearer imaging of fine internal battery features during thermal runaway events. Moreover, the insulation case was designed to provide system fire-proofing for both the chamber and pouch cell testing case configurations.
Lastly, a control switchbox is also being developed to work with the latest generation calorimeter. It allows users to remotely operate the TR trigger mechanism from a control room, automatically terminate power in a prescribed amount of time to prevent a fire caused by overheating, and provides lit indicators to inform the user of ready or fault states.
Electrical and Electronics
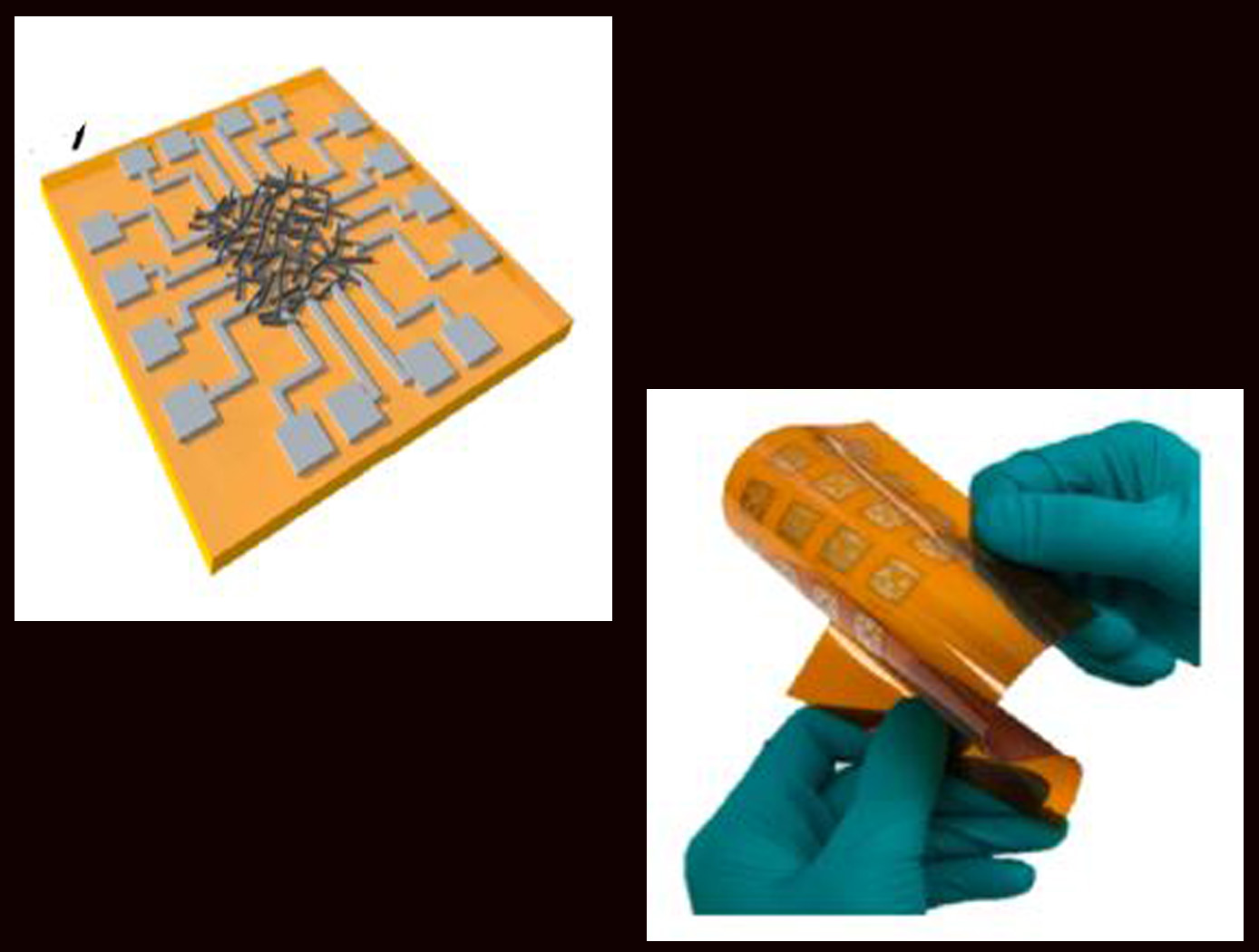
Highly secure all-printed Physically Unclonable Function (PUF) electronic device based on a nanomaterial network
The technology is an all-printed Physically Unclonable Function (PUF) electronic device based on a nanomaterial (such as single-walled carbon nanotube) network. The network may be a mixture of semiconducting and metallic nanotubes randomly tangled with each other through the printing process. The all-printed PUF electronic device comprises a nanomaterial ink that is inkjet deposited, dried, and randomly tangled on a substrate, creating a network. A plurality of electrode pairs is attached to the substrate around the substrate perimeter. Each nanotube in the network can be a conduction path between electrode pairs, with the resistance values varying among individual pairs and between networks due to inherent inter-device and intra-device variability. The unique resistance distribution pattern for each network may be visualized using a contour map based on the electrode information, providing a PUF key that is a 2D pattern of analog values. The PUF security keys remain stable and maintain robustness against security attacks. Although local resistance change may occur inside the network (e.g., due to environmental impact), such change has little effect on the overall pattern. In addition, when a network-wide resistance change occurs, all resistances are affected together, so that the unique pattern is maintained.
Health Medicine and Biotechnology

Portable Microscope
The handheld digital microscope features a 3D-printed chassis to house its hardware, firmware, and rechargeable Li-ion battery with built-in power management. It incorporates an internal stainless-steel cage system to enclose and provide mechanical rigidity for the optics and imaging sensor. To reduce the microscope’s size, yet retain high spatial resolution, engineers devised an optical light path that uniquely folds back on itself using high reflectivity mirrors, thus significantly reducing internal volume.
Imaging control and acquisition is performed using a secure web-based graphical user interface accessible via any wireless enabled device. The microscope serves as its own wireless access point thus obviating the need for a pre-existing network. This web interface enables multiple simultaneous connections and facilitates data sharing with clinicians, scientists, or other personnel as needed. Acquired images can be stored locally on the microscope server or on a removable SD card. Data can be securely downloaded to other devices using a range of industry standard protocols.
Although the handheld digital microscope was originally developed for in-flight medical diagnosis in microgravity applications, prototypes were thoroughly ground-tested in a variety of environments to verify the accurate resolve of microbial samples for identification and compo-sitional analysis for terrestrial field use. Owing to its portability, other applications demanding rapid results may include research, education, veterinarian, military, contagion disaster response, telemedicine, and point-of-care medicine.
Health Medicine and Biotechnology

Portable Slide Staining System for Microscopy
To stain a specimen slide, one or more liquid reagents are injected via the dispenser into the slide staining device via a syringe port. The volume of a given reagent is determined by adjustable settings on the dispenser, so that when connected to the staining device, initiates a thin film over the slide. The dispensing device uses only a fraction of the reagents typically used in non-sealed environments. Medical grade polyvinyl alcohol sponges have been incorporated into the dispenser to provide additional fluid containment and retention during the staining procedure. Furthermore, the dispenser can recall excess reagent, minimizing reagent use until refill.
The slide staining device is composed of an upper and lower section held together and aligned by use of Nd magnets. With the device open, a specimen slide is positioned upon a silicone gasket that sits within a recess in the lower section. When the device is closed, the silicone gasket in the upper section applies a seal to the slide forming a cavity that allows the slide to be exposed to reagents injected from the connected dispenser creating a stain through the use of capillary forces. Although originally designed for use in microgravity, the slide staining system also works in gravity environments.
Numerous applications may exist for this technology, particularly in hematology and cellular biology. Other applications could be considered for academic research, veterinary field use, military, disaster stricken and remote environments or where fine control of fluid delivery, removal, and management is desired.
The slide staining system is at technology readiness level (TRL) 8 (actual system completed and "flight qualified" through test and demonstration), and are now available to license. Please note that NASA does not manufacture products itself for commercial sale.



