Search
Health Medicine and Biotechnology

Cryogenic Oxygen Storage Modules COSM
The COSM employs NASA's Cryogenic Flux Capacitor core to store liquid oxygen (at 90 K) in silica aerogel material at ambient pressure, and then discharges cold oxygen gas into an in-line flow loop in response to heat input. If the composition of the incoming effluent stream contains gases with condensation or freezing points above the 90 K oxygen storage temperature--such as carbon dioxide or water vapor--these gasses can be removed from the stream as it moves through the COSM. The current COSM is sized to be wearable on the person but can be easily scaled to much larger sizes and various geometries.
COSM is designed with a long "cold path" which provides for greater residence times which increase the probability that condensable/freezable gases will be trapped in the COSM. Also, the longer the cold path, the longer the time a COSM can be used prior to the oxygen being depleted and the scrubbed gasses liberated. Two COSM geometries have been designed, built, and tested-a round spiral and a prismatic serpentine--to achieve long cold paths, and intrinsic vapor cooling to manage heat loads.
materials and coatings
Diamond Maker Technology Simulates Alien Geology in Laboratories
Given the significant impact of fO2 on material properties, it is important to perform studies at fO2 values relevant to the sample of interest. However, current systems used to control fO2 in solid media assemblies (e.g., sliding sensors, graphite capsule buffering) have limited ability to produce the wide fO2 ranges necessary to simulate more extreme planetary interiors.
NASA's fO2 control system implements a modified double capsule design that utilizes a wide range of solid metal-oxide buffers to control fO2 across a wide range of conditions. The approach is a modification of the previous double capsule design wherein the outer capsule itself acts as the metal component of the buffer assemblage, and the inner capsule to which the astromaterial sample of interest is placed resides within the outer capsule.
To achieve higher experimental temperatures above the melting point of more traditional noble metal capsule materials, outer capsules of the control assembly are comprised of refractory (high melting point) metals such as Ni, Co, W, Fe, Mo, V, Cr, Nb and Ta. Resultant experiments have reliably achieved temperatures exceeding 1600 degrees C, and when used in conjunction with a multi-anvil press, system pressures of over 20 GPa can be obtained lending researchers the ability to simulate on or off-world geologic conditions previously difficult to obtain in a laboratory.
NASA's fO2 control system was developed to enable high pressure, high temperature experimental studies of astromaterials at fO2 values relevant to the sample of interest. However, it may also be useful for the synthesis of materials where fO2 control is required (e.g., synthesis of crystal structures that might be stable under higher oxygen pressure). Further use cases may include mineral or melt syntheses, metal-silicate or mineral-melt element partitioning, phase equilibria studies, and the possible development of new chemical and mineral compounds that could not be manufactured in laboratories before.
This fO2 control system technology is at a technology readiness level (TRL) 6 (system/subsystem prototype demonstration in a relevant environment). The innovation is now available for your company to license. Please note that NASA does not manufacture products itself for commercial sale.
sensors
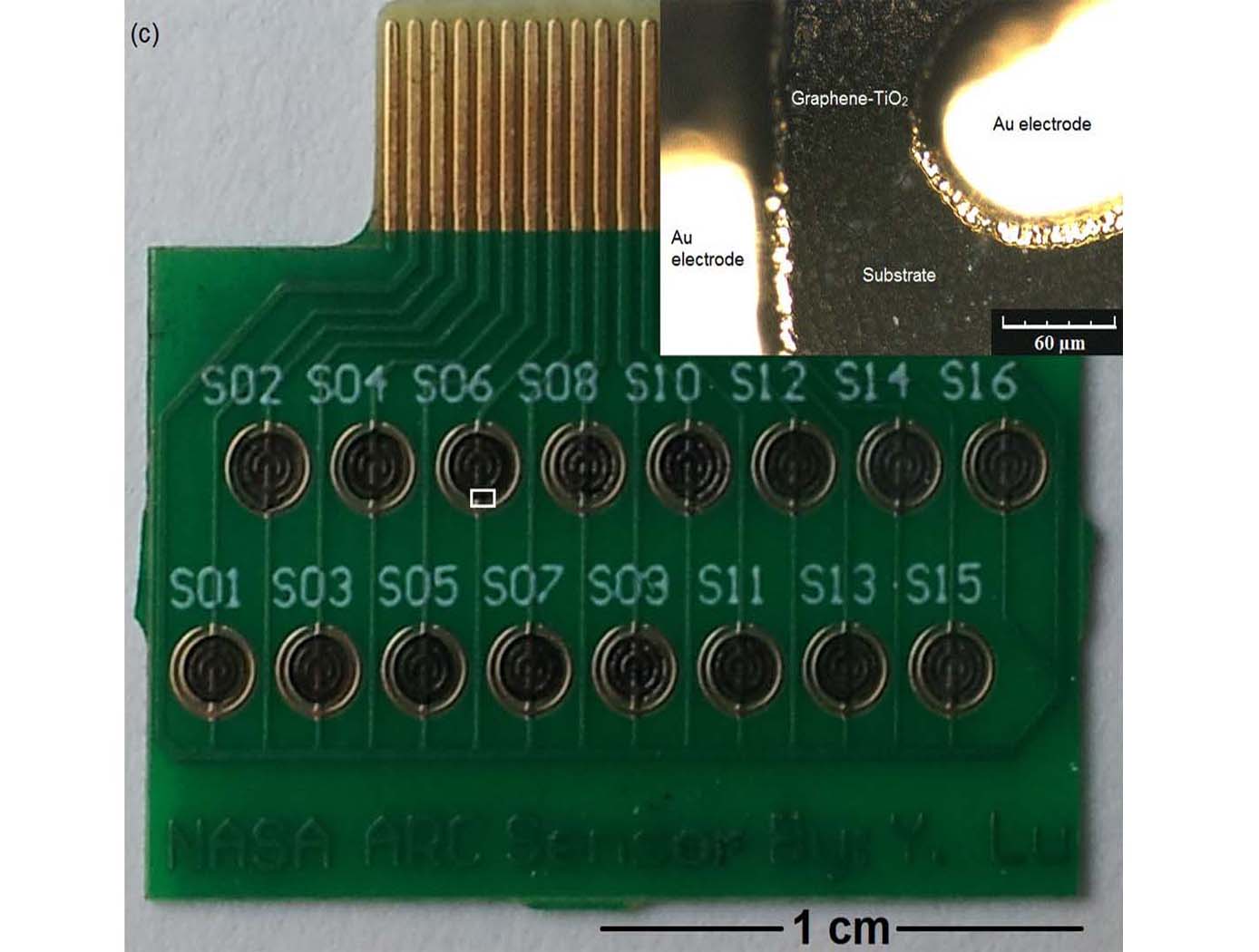
Room temperature oxygen sensors
NASA Ames has developed very small-sized oxygen sensors made of a graphene and titanium dioxide (TiO<sub>2</sub>) hybrid material. With ultraviolet (UV) illumination, these sensors are capable of detecting oxygen (O<sub>2</sub>) gas at room temperature and at ambient pressure. The sensors are able to detect oxygen at concentrations ranging from about 0.2% to about 10% by volume under 365nm UV light, and at concentrations ranging from 0.4% to 20% by volume under short wave 254nm UV light. These sensors have fast response and recovery times and can also be used to detect ozone.
This unique room temperature O<sub>2</sub> sensor provides significant advantages in O<sub>2</sub> sensing applications, especially those applications where high operating temperature requirements cannot be met, or would result in inefficient manufacturing processes. Since graphene is not intrinsically responsive to O<sub>2</sub>, and TiO<sub>2</sub> is not responsive to oxygen at room temperature, the materials are first synthesized as a hybrid material. The synthesized graphene- TiO<sub>2</sub> hybrid material is then ultrasonicated and then drop-casted onto a series of Interdigitated Electrodes (IDE) to form the sensors.
Ultrasonication ensures effective charge transfer at the graphene- TiO<sub>2</sub> interphase. The graphene and the titanium dioxide may be present in the composite material in different ratios to ensure optimal oxygen detection. It is the combination of graphene with TiO2 that yields a semiconducting material capable of O<sub>2</sub> sensing at room-temperature operation.
Environment

Air Revitalization for Vacuum Environments
The NASA life support system uses a regenerable vacuum swing adsorption process, known as Sorbent-Based Air Revitalization (SBAR), to separate water and carbon dioxide for disposal. The SBAR system is an adsorbent-based swing bed system that has been optimized to provide both humidity and carbon dioxide control for a spacecraft cabin atmosphere.
The system comprises composite silica gel and zeolite-packed beds for adsorption and a bypass system for flow control. Under normal operating conditions, the disposal system would require a high-quality vacuum environment to operate. Improvements to the SBAR system include an enhanced inherent capacitance that extends the operation time within a non-vacuum environment for up to 4.5 hours. Flight time can be further expanded with multiple SBAR systems to allow for system regeneration. By scheduling periodic thermal regenerations—nominally during sleep periods—the SBAR technology may be suitable for missions of unlimited duration.
Power Generation and Storage

Cryogenic Flux Capacitor
Storage and transfer of fluid commodities such as oxygen, hydrogen, natural gas, nitrogen, argon, etc. is an absolute necessity in virtually every industry on Earth. These fluids are typically contained in one of two ways; as low pressure, cryogenic liquids, or as a high pressure gases. Energy storage is not useful unless the energy can be practically obtained ("un-stored") as needed. Here the goal is to store as many fluid molecules as possible in the smallest, lightest weight volume possible; and to supply ("un-store") those molecules on demand as needed in the end-use application. The CFC concept addresses this dual storage/usage problem with an elegant charging/discharging design approach.
The CFC's packaging is ingeniously designed, tightly packing aerogel composite materials within a container allows for a greater amount of storage media to be packed densely and strategically. An integrated conductive membrane also acts as a highly effective heat exchanger that easily distributes heat through the entire container to discharge the CFC quickly, it can also be interfaced to a cooling source for convenient system charging; this feature also allows the fluid to easily saturate the container for fast charging. Additionally, the unit can be charged either with cryogenic liquid or from an ambient temperature gas supply, depending on the desired manner of refrigeration. Finally, the heater integration system offers two promising methods, both of which have been fabricated and tested, to evenly distribute heat throughout the entire core, both axially and radially.
NASA engineers also applied the CFC to a Cryogenic Oxygen Storage Module to store oxygen in solid-state form and deliver it as a gas to an end-use environmental control and/or life support system. The Module can scrub out nuisance or containment gases such as carbon dioxide and/or water vapor in conjunction with supplying oxygen, forming a synergistic system when used in a closed-loop application. The combination of these capabilities to work simultaneously may allow for reduced system volume, mass, complexity, and cost of a breathing device.
Environment

Modular Container System Preserves Sample Integrity
The Astromaterials Acquisition and Curation Office (AACO) at NASA Johnson Space Center currently curates 500 milligrams of the regolith sample from the Asteroid Ryugu that was collected by the Japan Aerospace and Exploration Agency’s Hayabusa II spacecraft and returned to Earth in 2021. In September 2023, NASA’s OSIRIS-REx spacecraft returned 70 grams of regolith collected from the surface of Asteroid Bennu. These astromaterial sample collections are stored and handled in gloveboxes and desiccators that are continuously purged with ultrapure nitrogen in order to minimize contamination and alteration of extraterrestrial samples from terrestrial environments.
For collaborative astromaterial sample research conducted outside of the AACO, a need emerged for a sample container system suitable for global transport, capable of maintaining the same low-oxygen envi-ronment as laboratory gloveboxes. Thus, the MCS was developed. MSC’s of different sizes (2, 4, and 8-inch sample container models) have been developed to store contact pads and bulk samples from NASA missions, including the OSIRIS-REx Asteroid Bennu mission.
MCS’s are designed with seal profiles to prevent oxygen from seeping into the sample container. Additionally, the MCS uses a sample container form-factor that optimizes favorable nitrogen to oxygen gas ratios. The final prototypes were tested and verified using optochemical sensors to measure trace oxygen levels within the sealed containers.
The Modular Container System (MCS) could fill a critical gap in the existing high-purity logistics and storage market in its ability to provide a passively maintained, verifiable, multi-year, glovebox-level low-oxygen environment in a portable robust form-factor. Although this technology was originally developed for astromaterial transport and storage, commercial applications may also exist in biopharmaceutical/ bio-banking, microelectronics/ semiconductor, and other industries.



