SABERS: Solid-State Lithium-Sulfur Battery Technology Portfolio
Power Generation and Storage
SABERS: Solid-State Lithium-Sulfur Battery Technology Portfolio (LEW-TOPS-167)
New Battery Paradigm for Energy Density, Power, Reliability, and Safety
Overview
SABERS (Solid-state Architecture Batteries for Enhanced Rechargeability and Safety) is a portfolio of innovations developed collaboratively by NASA's Glenn, Langley, and Ames Research Centers that addresses fundamental challenges in solid-state battery technology. Solid-state batteries replace the flammable liquid electrolytes found in conventional lithium-ion batteries with solid materials, offering transformative improvements in safety and energy density. However, realizing this potential requires solving critical problems in materials compatibility, manufacturing scalability, and system integration. SABERS tackles these challenges through several complementary innovations: novel materials, graphene-based manufacturing, dry-processing techniques, solid-state architecture developments, and computational design tools.
NASA developed SABERS primarily for next-generation electric aviation propulsion, where solid-state batteries' combination of high energy density, damage tolerance, and inherent safety is essential. The technology addresses broader challenges facing solid-state battery commercialization across automotive, aerospace, energy storage, and other industries. NASA is seeking industry partners to advance this technology toward commercial deployment. SABERS is available for licensing as a complete portfolio or as individual component technologies.
The Technology
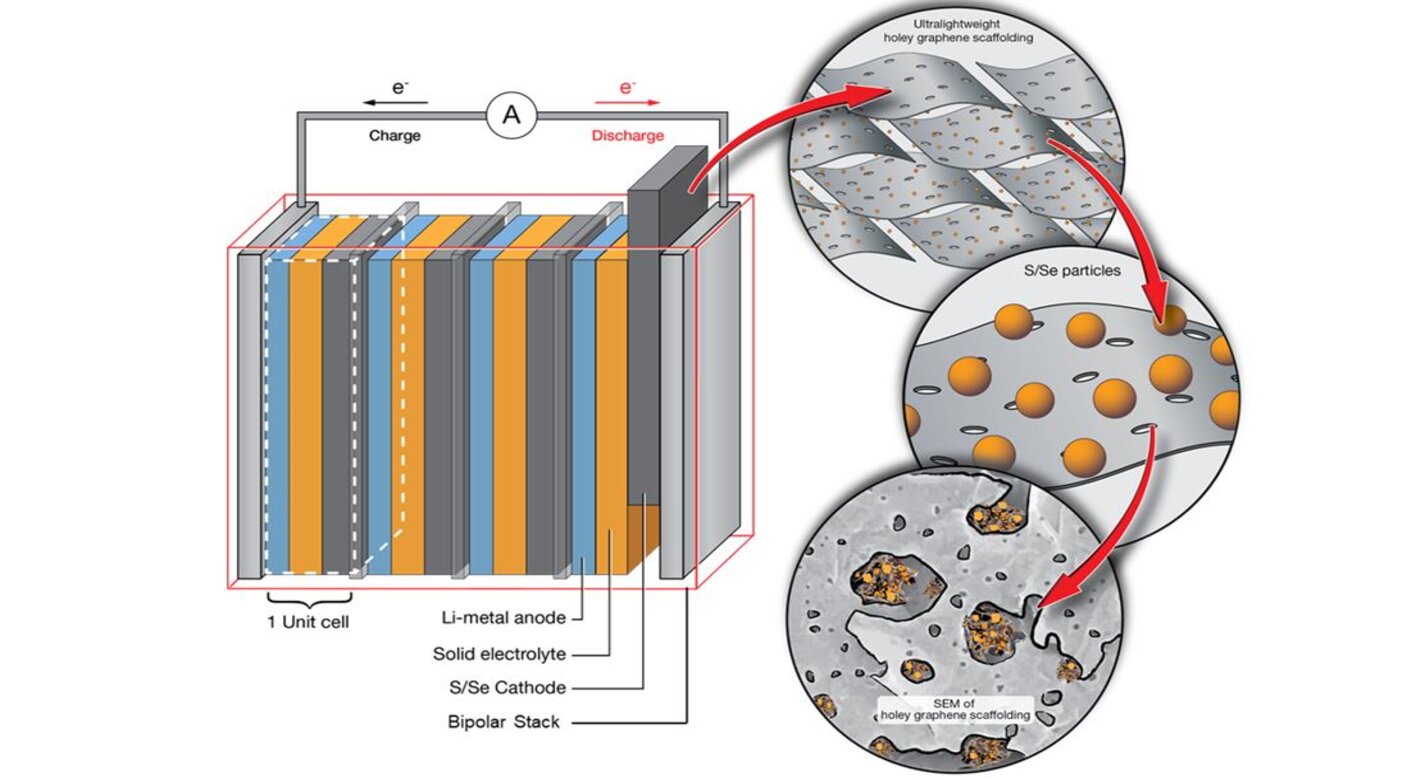
The SABERS innovators developed novel lithium-sulfur designs, including sulfur-selenium on graphene cathodes, and lightweight bipolar plate stacking and packaging designs. SABERS is unique in several aspects, in particular, it deploys graphene-based manufacturing processes for the cathode and bipolar plates, and it uses a solid-state electrolyte in place of the liquid electrolyte found in other lithium-sulfur battery designs. The team has achieved energy densities over 500 W-hr/kg, with ongoing development targeting further improvements. Coin cell and pouch prototype demonstrations have been successful and are ongoing.
Major component technologies in SABERS include the following:
• S/Se Cathode – Sulfur/Selenium on graphene scaffold (LEW-20228-1)
• Solid Electrolyte – Solid-state electrolyte composites (LEW-20445-1)
• Bipolar Stack – Graphene plates (LAR-20257-1)
Robust computational models have been developed to support the battery materials design and are available to licensees to evaluate and optimize different materials combinations and performance targets.
Further developments in catholyte formulations, anode interlayering, and packaging optimization are presented in SABERS 2.0 (LEW-TOPS-188). Individual technologies can be licensed from either suite, or entire portfolios can be licensed to support solid-state battery development programs.
Benefits
- High Power Capability: Designed for high discharge rates necessary for aircraft takeoff and rapid vehicle acceleration, with robust performance maintained under demanding power loads.
- Exceptional Safety: Solid-state electrolyte eliminates flammable liquid components, providing high damage tolerance and eliminating thermal runaway and fire risks even under penetration, crushing, or short-circuit conditions.
- Lightweight Design: Architecture and component developments reduce inactive mass by 30-40% compared to conventional designs while maintaining structural robustness.
- Scalable Manufacturing: Graphene-based processing and novel dry-processing techniques eliminate costly solvent-based techniques, reduce production time and energy consumption, and enable ready scaling to commercial manufacturing volumes.
- Prototype Demonstration: Coin-cell and pouch type batteries have been successfully demonstrated, and research is still ongoing to optimize design and performance.
- Environmentally Sustainable: Uses earth-abundant sulfur/selenium active materials and solvent-free manufacturing processes that reduce environmental impact.
Applications
- Electric Aviation: Battery-powered propulsion systems for next-generation electric aircraft, urban air mobility vehicles, and long-endurance drones, enabling zero-emission flight and transforming aerospace transportation.
- Automotive: Lightweight batteries offer improved crash safety, sustainability, and driving range.
- Spacecraft and Satellites: Enhanced endurance, reliability, and energy storage for spaceflight applications, reducing the need for servicing and refueling in mission-critical systems.
- Defense and Military: High-performance batteries for unmanned systems, portable power for soldiers, and mission-critical applications where weight, energy density, safety, and reliability are paramount.
- Grid-Scale Energy Storage: Advanced energy storage systems for renewable energy integration with higher energy density that reduces land requirements and eliminates fire risks associated with large lithium-ion installations.
Technology Details
Power Generation and Storage
LEW-TOPS-167
LEW-20228-1
LEW-20445-1
Dry Pressing Neat Active Materials into Ultrahigh Mass Loading Sandwich Cathodes Enabled by Holey Graphene Scaffold, https://pubs.acs.org/doi/full/10.1021/acsaem.0c00582
Holey Graphene–Enabled Solvent-Free Preparation of Ultrahigh Mass Loading Selenium Cathodes for High Areal Capacity Lithium–Selenium Batteries, https://www.frontiersin.org/articles/10.3389/fenrg.2021.703676/full
Practical considerations in designing solid state Li-S cells for electric aviation, https://www.sciencedirect.com/science/article/abs/pii/S0013468621016960
Li-Ion Permeability of Holey Graphene in Solid State Batteries: A Particle Dynamics Study https://pubs.acs.org/doi/full/10.1021/acsami.2c03012
Relevant NTR numbers for the SABERS suite of technology include: LAR-19556-1, LEW-20228-1, LAR-18334-1, LEW-20445-1, LAR-20257-1, LAR-19842-1, LAR-18867-1, LAR-18867-2, LAR-18334-1, LEW-20610-1, LEW-20611-1, LEW-20638-1, LAR-20546-1
Holey Graphene–Enabled Solvent-Free Preparation of Ultrahigh Mass Loading Selenium Cathodes for High Areal Capacity Lithium–Selenium Batteries, https://www.frontiersin.org/articles/10.3389/fenrg.2021.703676/full
Practical considerations in designing solid state Li-S cells for electric aviation, https://www.sciencedirect.com/science/article/abs/pii/S0013468621016960
Li-Ion Permeability of Holey Graphene in Solid State Batteries: A Particle Dynamics Study https://pubs.acs.org/doi/full/10.1021/acsami.2c03012
Relevant NTR numbers for the SABERS suite of technology include: LAR-19556-1, LEW-20228-1, LAR-18334-1, LEW-20445-1, LAR-20257-1, LAR-19842-1, LAR-18867-1, LAR-18867-2, LAR-18334-1, LEW-20610-1, LEW-20611-1, LEW-20638-1, LAR-20546-1
Similar Results

Carbon Bipolar Membranes for Solid-State Batteries
In traditional batteries with liquid electrolytes, e.g., lithium-ion, each battery cell must be individually sealed, packaged, and electrically connected to other cells in the pack. The cells in solid-state batteries on the other hand may be stacked on top of one another with only a separation layer in between, called a bipolar plate. These bipolar plates or membranes if thin enough must be electrochemically inert to the electrode and electrolyte materials while providing electrical connectivity between the individual cells.
Here, NASA has combined advances in the preparation of carbon nanomaterials and solid-state batteries to create extremely lightweight bipolar plates and membranes. These bipolar membranes will enable high energy density solid-state batteries unachievable with typical bipolar plate materials like stainless steel, aluminum, aluminum-copper, or conductive ceramics. The carbon bipolar membranes may be fabricated in multiple ways including but not limited to directly compressing carbon powders onto an electrode-electrolyte stack or separately making a film of the carbon material and dry pressing the film between other battery layers. The new bipolar membranes have been demonstrated in high energy density solid-state batteries in coin and pouch cells.
The carbon bipolar membranes are at technology readiness level TRL-4 (Component and or breadboard validation in laboratory environment)and are available for patent licensing.

Holey Carbon Allotropes
This invention is for scalable methods that allows preparation of bulk quantities of holey nanocarbons with holes ranging from a few to over 100 nm in diameter. The first method uses metal particles as a catalyst (silver, copper, e.g.) and offers a wider range of hole diameter. The second method is free of catalysts altogether and offers more rapid processing in a single step with minimal product work-up requirements and does not require solvents, catalysts, flammable gases, additional chemical agents, or electrolysis. The process requires only commercially available materials and standard laboratory equipment; and, it is scalable. Properties that can be controlled include: surface area, pore volume, mechanical properties, electrical conductivity, and thermal conductivity.

Electrolyte for Aluminum-Air Batteries
Aluminum-air batteries produce electricity from the reaction of atmospheric oxygen with aluminum. They have extremely high energy densities, but significant problems remain with byproduct removal due to use of traditional electrolytes. The electrolyte used is an aqueous potassium hydroxide (KOH) solution, incorporated into a polymer-based electrolyte matrix.
Traditional alkaline electrolytes enable high ionic conductivity but corrode aluminum, wasting active material and releasing hydrogen gas. Unlike free liquid electrolytes, this hybrid design holds the conductive solution in place, providing the same high ionic conductivity while dramatically reducing the uncontrolled corrosion and gas evolution that typically deplete aluminum electrodes. The polymer host also prevents leakage and drying, improving reliability under demanding conditions such as high altitude and variable temperature environments.
The aluminum-air battery electrolyte is a lightweight, high-capacity, and inherently safer primary power source that can meet stringent aerospace requirements for emergency and backup energy. Beyond aircraft, the technology’s combination of high energy density, safety, and sustainable byproducts makes it attractive for electric aircraft, defense systems, and other mission-critical applications. The electrolyte for aluminum-air batteries is available for patent licensing.

Room temperature oxygen sensors
NASA Ames has developed very small-sized oxygen sensors made of a graphene and titanium dioxide (TiO2) hybrid material. With ultraviolet (UV) illumination, these sensors are capable of detecting oxygen (O2) gas at room temperature and at ambient pressure. The sensors are able to detect oxygen at concentrations ranging from about 0.2% to about 10% by volume under 365nm UV light, and at concentrations ranging from 0.4% to 20% by volume under short wave 254nm UV light. These sensors have fast response and recovery times and can also be used to detect ozone.
This unique room temperature O2 sensor provides significant advantages in O2 sensing applications, especially those applications where high operating temperature requirements cannot be met, or would result in inefficient manufacturing processes. Since graphene is not intrinsically responsive to O2, and TiO2 is not responsive to oxygen at room temperature, the materials are first synthesized as a hybrid material. The synthesized graphene- TiO2 hybrid material is then ultrasonicated and then drop-casted onto a series of Interdigitated Electrodes (IDE) to form the sensors.
Ultrasonication ensures effective charge transfer at the graphene- TiO2 interphase. The graphene and the titanium dioxide may be present in the composite material in different ratios to ensure optimal oxygen detection. It is the combination of graphene with TiO2 that yields a semiconducting material capable of O2 sensing at room-temperature operation.

Novel, Solid-State Hybrid Ultracapacitor Battery
The subject technology is an extension of closely related, solid-state ultracapacitor innovations by the same team of inventors. The primary distinction for this specific technology is the addition of co-dopants to affect the dielectric behavior of the barium titanatebased perovskite materials. These co-dopants include lanthanum and other rare earths as well as hydroxyl ions. The materials are processed at the nano scale, and are subjected to carefully designed thermal treatments as well.
The presence of the hydroxyl ions has been shown to provide several orders of magnitude increase in the capacitance of the dielectric material. Additionally, these high capacitance values are obtained at relatively low voltages found in current consumer and industrial electronics.
The capacitors tested to date are simple, single-layer devices. Ultimately, a range of manufacturing methods are possible for making commercial devices. Features of the technology enable manufacturing via traditional thick-film processing methods widely used in the capacitor industry, or via advanced printing methods for state-of-the-art printed electronics.
Future efforts will be made to advance the manufacturing and packaging processes to increase device energy density, including multilayer devices and packages



